Abstract
Background:
The presence of measurable residual disease (MRD) is strongly associated with treatment outcomes in acute myeloid leukemia (AML). However, > 20% of MRD negative cases (as assessed by flow cytometry) subsequently relapse. We sought to determine if ultrasensitive Duplex Sequencing (DS), which relies on double-stranded consensus-making to achieve an error rate below one-in-ten-million, yields better prognostic performance via molecular MRD detection.
Methods:
Retrospective targeted DNA sequencing of 29 genes recurrently mutated in adult AML was performed on paired diagnostic and remission bone marrow samples from patients enrolled on the SWOG trial S0106 (randomized 7+3 versus 7+3 + gemtuzumab ozogamicin (GO)). Patients were selected if they had remission samples with flow cytometry results (n=67). Non-error corrected sequencing was performed on diagnostic samples (average depth 279x) and DS was performed on remission samples (average duplex molecular depth 27,002x). For each patient, potential germline variants were identified and excluded from the analysis if the variant allele fraction (VAF) was ≥ 35% at both diagnosis and remission, or ≥ 40% at either time point and a gnomAD allele frequency ≥ 0.05. Somatic variants present at diagnosis were classified as potentially deleterious if computationally predicted as such and with a VAF ≥ 5% (≥ 1% for FLT3-ITD/NPM1 insertions). For analysis of residual disease in remission, we evaluated the following outcomes (events): overall survival (OS; death), relapse-free survival (RFS) and time to relapse (TTR; relapse with death a competing event). All outcomes were measured from date of morphologic remission to date of event, with patients without event censored at date of last contact. Associations between residual disease and outcomes were assessed using Cox regression models (cause-specific model for TTR).
Results:
The median age was 48 years (range 8-60). 32 patients were randomized to 7+3 and 30 to 7+3+GO. A total of 172 potentially deleterious variants were identified in the diagnostic samples. Variants had an average VAF of 31% (range 1.4-91.5%) at diagnosis and were detected in 23 of the 29 genes, with FLT3 being the most frequently mutated. Of the 67 patients analyzed, 93% (n=62) had at least one variant detected at diagnosis (median 2, range 0-9) and 68% (n=42) had at least one residual diagnostic variant also found in the remission sample.
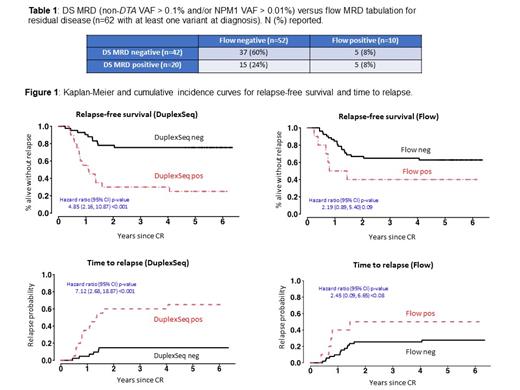
We defined the presence of DS MRD as non-DTA (DNMT3A, TET2, ASXL1) time-of-diagnosis mutations identified at the remission time point with a VAF > 0.1% and/or an NPM1 VAF > 0.01% (PMID:31860405). DS MRD was strongly associated with all outcomes, with hazard ratios (and 95% CI) for TTR: 7.1 (2.7-18.9); RFS: 4.9 (2.2-10.9) and OS: 5.1 (2.1-12.3).
As a comparator, we correlated treatment outcomes with the results of a flow cytometry MRD assay previously carried out on the same samples during the S0106 trial. The prognostic association of flow MRD with TTR, RFS and OS was less strong (i.e., a smaller hazard ratio) than DS MRD, with TTR: 2.5 (0.9-6.7); RFS: 2.2 (0.9-5.4) and OS: 2.4 (1.0-6.1). RFS and TTR for DS MRD and flow cytometry are plotted below for the 62 patients with a variant detected at diagnosis.
Comparing DS MRD with flow cytometry, discordance was found in 20 cases: 15 cases where DS was positive and flow negative, and 5 cases in the opposite direction. Among the 15 discordant cases with DS positive and flow negative, 9 relapsed and 2 died without relapse and 4 were alive without relapse at last contact. Among the 5 discordant cases with DS negative and flow positive, 1 relapsed, 1 died without relapse, and 3 were alive without relapse at last contact.
Conclusions:
Among the 67 patients evaluated in this prospectively collected study, the presence of MRD defined by DS was strongly associated with adverse disease outcomes. The vast majority of patients had at least one time-of-diagnosis mutation that could be tracked as a measure of MRD. When compared with flow cytometry, DS exhibited superior negative and positive predictive values for foretelling future relapse, although this could potentially reflect the historical version of the flow cytometry assay used. These findings suggest that DS is a powerful tool that could be used in patient management and for early treatment assessment in clinical trials.
Hourigan: Sellas: Research Funding. Radich: Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.